- Raybet比分 提供学术活动、国际课程、科研项目一站式留学背景提升服务!
- 400 888 0080
CIE A Level Chemistry复习笔记2.3.2 Chemical Properties: Halogens & Hydrogen Halides
Group 17: Oxidising Agents
- Halogens react with metals by accepting an electron from the metal atom to become an ion with 1- charge
Eg. Ca(s) + Cl2(g) → Ca2+(Cl-)2(s)
- Halogens are therefore oxidising agents:
- Halogens oxidise the metal by removing an electron from the metal (the oxidation number of the metal increases)
- Halogens become reduced as they gain an extra electron from the metal atom (the oxidation number of the halogen decreases)
- The oxidising power of the halogens decreases going down the group (the halogens get less reactive)
- This can be explained by looking at their electronegativities:
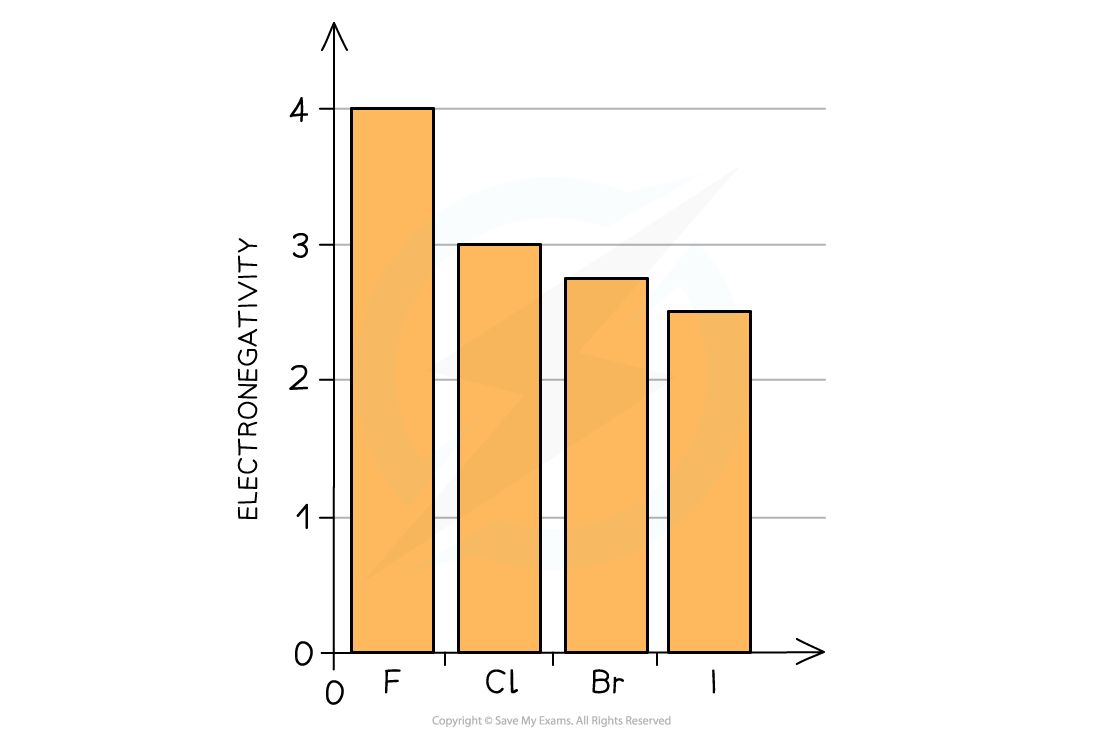
The electronegativity of the halogens decreases going down the group
- The electronegativity of an atom refers to how strongly it attracts electrons towards itself in a covalent bond
- The decrease in electronegativity is linked to the size of the halogens
- Going down the group, the atomic radii of the elements increase which means that the outer shells get further away from the nucleus
- An ‘incoming’ electron will therefore experience more shielding from the attraction of the positive nuclear charge
- The halogens’ ability to accept an electron (their oxidising power) therefore decreases going down the group
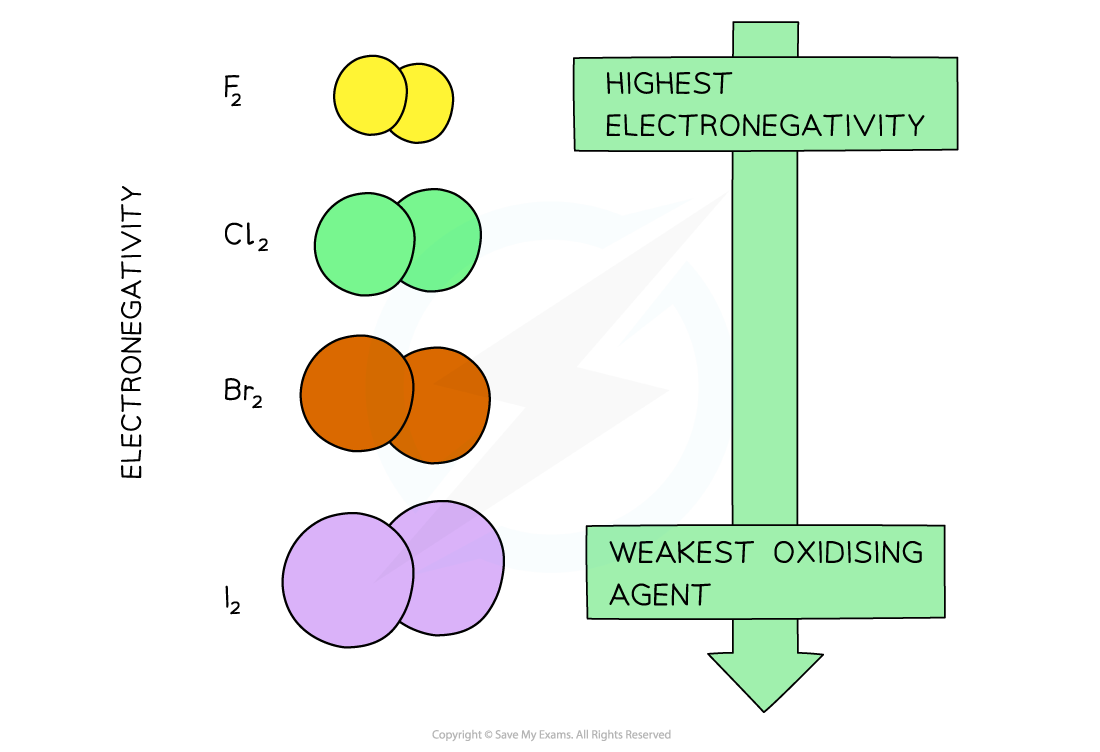
With increasing atomic size of the halogens (going down the group) their electronegativity, and therefore oxidising power, decreases
- The reactivity of halogens is also shown by their displacement reactions with other halide ions in solutions
- A more reactive halogen can displace a less reactive halogen from a halide solution of the less reactive halogen
- Eg. The addition of chlorine water to a solution of bromine water:
Cl2(aq) + 2NaBr(aq) → 2NaCl(aq) + Br2(aq)
-
- The chlorine has displaced the bromine from solution as it is more reactive which can be summarised in the following ionic equation:
Cl2(aq) + 2Br-(aq) → 2Cl-(aq) + Br2(aq)
Group 17: Reaction with Hydrogen
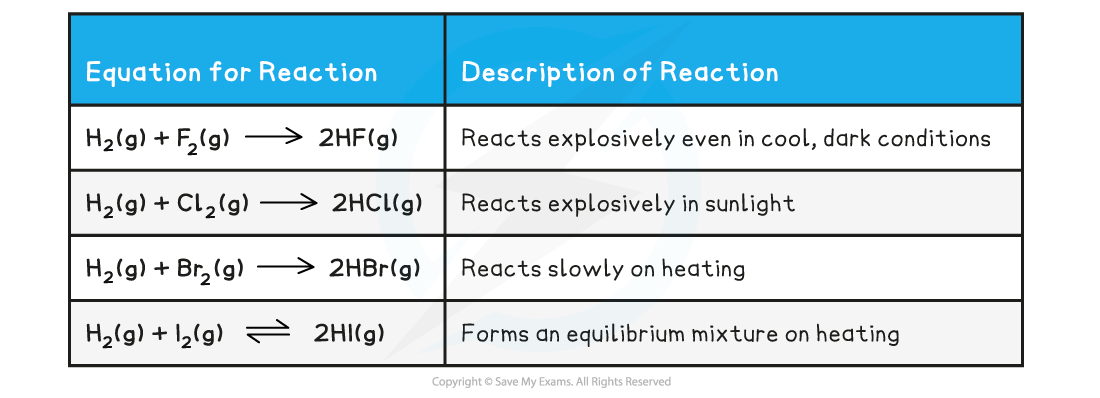
- Halogens react with hydrogen gas to form hydrogen halides
- Due to the decrease in reactivity of the halogens going down the group, the reactions between halogen and hydrogen gas become less vigorous
- The table below shows a summary of the reaction between halogen and hydrogen gas
Reaction between halogen & hydrogen gas
Thermal Stability of the Hydrogen Halides
- Thermal stability refers to how well a substance can resist breaking down when heated
- A substance that is thermally stable will break down at high temperatures
- The hydrogen halides formed from the reaction of halogen and hydrogen gas decrease in thermal stability going down the group
- The decrease in thermal stability can be explained by looking at the bond energies of the hydrogen-halogen bond
- Going down the group, the atomic radius of the halogens increases
- The overlap of its outer shell with a hydrogen atom therefore gives a longer bond length
- The longer the bond, the weaker it is, and the less energy required to break it
- As the bonds get weaker, the hydrogen halogens become less stable to heat going down the group
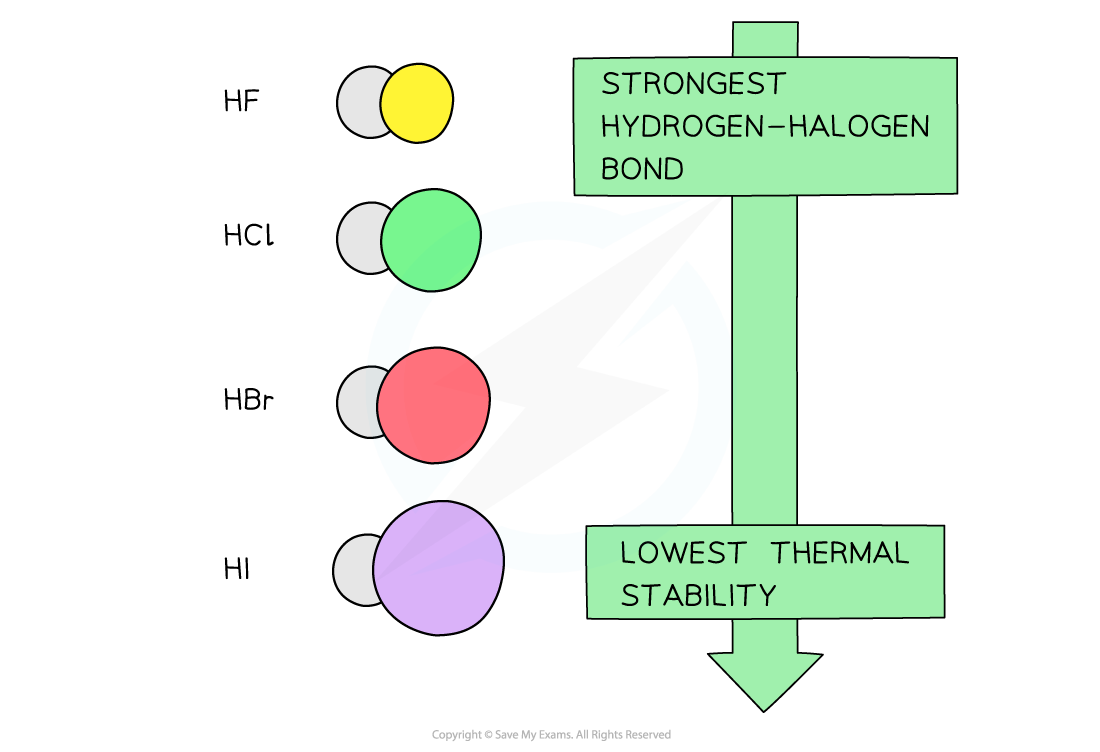
The thermal stability of the hydrogen halide decreases going down the group as their bonds become weaker due to the increased atomic radius of the halogens
转载自savemyexams
在线登记
最新发布
Raybet比分 课程体验,退费流程快速投诉邮箱: yuxi@linstitute.net 沪ICP备2023009024号-1